
Viral Vector Manufacturing Market Anticipated to Reach USD 6.5 Billion, at a Notable 11.94% CAGR by 2034
Viral Vector Manufacturing Market
The market is witnessing strategic partnerships, mergers, and acquisitions aimed at strengthening manufacturing capabilities.
US, NY, UNITED STATES, March 11, 2025 /EINPresswire.com/ -- The viral vector manufacturing market has witnessed significant growth in recent years, driven by the increasing demand for gene therapy, cell therapy, and vaccine production. Viral vectors, including adenoviruses, lentiviruses, and adeno-associated viruses (AAV), serve as essential tools in gene transfer, enabling the treatment of genetic disorders, cancers, and infectious diseases. As research and clinical applications continue to expand, the market is experiencing dynamic changes fueled by technological advancements and regulatory support.
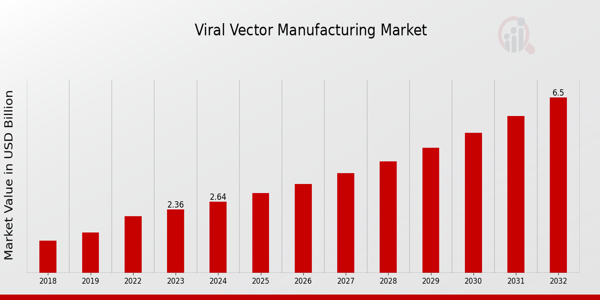
As per MRFR analysis, the Viral Vector Manufacturing Market Size was estimated at 2.1 (USD Billion) in 2022. The Viral Vector Manufacturing Market Industry is expected to grow from 2.36 (USD Billion) in 2023 to 6.5 (USD Billion) by 2032. The Viral Vector Manufacturing Market CAGR (growth rate) is expected to be around 11.94% during the forecast period (2024 - 2032).
Get a free sample here@ https://www.marketresearchfuture.com/sample_request/6890
Market Trends
Rising Demand for Gene and Cell Therapies
The increasing prevalence of genetic and rare diseases has boosted the demand for gene and cell therapies. With the approval of groundbreaking treatments such as Zolgensma and Luxturna, viral vector-based therapies are gaining traction. This trend is driving investments in viral vector manufacturing capacities globally.
Adoption of Advanced Bioprocessing Technologies
Manufacturers are adopting single-use bioreactors, automation, and modular facilities to enhance scalability and efficiency. Continuous manufacturing techniques and improved purification processes are helping streamline production, reducing costs and improving yields.
Expansion of Contract Manufacturing Organizations (CMOs)
Many biotech and pharmaceutical companies are outsourcing viral vector production to CMOs with specialized expertise. This trend is driven by the need for high-quality production, regulatory compliance, and cost reduction, allowing companies to focus on research and development.
Growing Investment and Collaborations
The market is witnessing strategic partnerships, mergers, and acquisitions aimed at strengthening manufacturing capabilities. Leading players are investing in expanding their facilities and collaborating with research institutions to accelerate innovation in viral vector production.
Regulatory Advancements and Support
Regulatory agencies, including the FDA and EMA, are providing clear guidelines to streamline the approval process for viral vector-based therapies. Standardization of Good Manufacturing Practices (GMP) is enhancing product quality and ensuring safety in clinical applications.
Innovations in Viral Vector Manufacturing
Improved Vector Engineering
Advancements in vector engineering have led to the development of more efficient and safer viral vectors with enhanced transduction efficiency, lower immunogenicity, and targeted gene delivery.
Scalable Manufacturing Platforms
Innovative scalable platforms, such as suspension-based culture systems, are replacing traditional adherent cell cultures, allowing for higher production yields and cost-effectiveness.
Artificial Intelligence (AI) and Automation
AI-driven bioprocessing and automation technologies are optimizing viral vector production by improving monitoring, predictive analytics, and process control, thereby enhancing quality and efficiency.
Next-Generation Purification Techniques
New purification strategies, such as chromatography-based techniques and advanced filtration methods, are improving the yield and purity of viral vectors, minimizing contamination risks.
CRISPR-Based Enhancements
CRISPR technology is being integrated into viral vector design to enhance gene editing efficiency and precision, paving the way for more targeted and effective therapies.
Growth Drivers
Increasing Prevalence of Genetic Disorders and Infectious Diseases
The rising burden of genetic diseases, such as muscular dystrophy and hemophilia, and the need for effective treatments for infectious diseases, such as COVID-19, are major drivers of market growth.
Rising Funding and Government Support
Governments and private investors are heavily funding gene therapy research, supporting biotech startups, and encouraging innovation in viral vector manufacturing.
Expansion of Vaccine Development
The COVID-19 pandemic highlighted the importance of viral vector-based vaccines, such as AstraZeneca and Johnson & Johnson’s COVID-19 vaccines. Ongoing vaccine research for emerging infectious diseases continues to drive market demand.
Increasing Clinical Trials and Approvals
A surge in clinical trials and the approval of novel gene therapies are contributing to market expansion. Several promising therapies are in late-stage clinical trials, boosting the demand for high-quality viral vector manufacturing.
Technological Advancements in Manufacturing
Innovations in bioprocessing, AI, and genetic engineering are enhancing the scalability, efficiency, and affordability of viral vector production, driving market growth.
Key Companies in the Viral Vector Manufacturing Market Include
Oxford Biomedica
Aldevron
Sangamo Therapeutics
Thermo Fisher Scientific
GenVec
Viralgen VectorCore
Adenovirus Center
Wuxi AppTec
Sarepta Therapeutics
VivaZome Therapeutics
Lonza
MaxCyte
Fujifilm Diosynth Biotechnologies
Charles River Laboratories
SIRION Biotech
Buy it now by visiting here: https://www.marketresearchfuture.com/checkout?currency=one_user-USD&report_id=6890
Viral Vector Manufacturing Market Segmentation Overview
Application Outlook:
Gene Therapy
Vaccines
Oncology
Cardiovascular Diseases
Type Outlook:
Adenoviral Vectors
Adeno-Associated Viral Vectors
Lentiviral Vectors
Retroviral Vectors
End-Use Outlook:
Pharmaceutical Companies
Research Institutions
Biotechnology Companies
Vector Design Outlook:
Self-Complementary
Single-Stranded
Double-Stranded
Regional Outlook:
North America
Europe
South America
Asia Pacific
Middle East & Africa
Related MRFR Reports with Full Detailed Analysis:
Androgenetic Alopecia Market- https://www.marketresearchfuture.com/reports/androgenetic-alopecia-market-26872
Animal Drug Compounding Market- https://www.marketresearchfuture.com/reports/animal-drug-compounding-market-40610
Animal Intestinal Health Market- https://www.marketresearchfuture.com/reports/animal-intestinal-health-market-40912
Antacid Market- https://www.marketresearchfuture.com/reports/antacid-market-8915
Anti-Fungal Drugs Market- https://www.marketresearchfuture.com/reports/anti-fungal-drugs-market-31940
Market Research Future
Market Research Future
+1 855-661-4441
email us here
Visit us on social media:
Facebook
X
LinkedIn
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release